About Soda Ash
Date:2020-04-20 Read:244
About Soda Ash

Soda ash, the trade name for sodium carbonate, Na2CO3, (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, water-soluble salts. All forms have a strongly alkaline taste and give moderately alkaline solutions in water.
Sodium carbonate has been used in manufacturing for over 5,000 years. Ancient Egyptians used it to make glass ornaments and vessels. They recovered the product from dry lake-bed deposits or by burning seaweed and other marine plants. The Romans also used soda ash for baking bread, making glass and for medicinal purposes. Its extraction from the ashes of various plants continued until the middle of the 19th century and gave it the present-day name of "soda ash".
Today, "natural soda ash" refined from the mineral trona is regarded as the standard for quality and purity and is also processed from sodium-carbonate-bearing brines. The Green River Basin of Wyoming is the world's largest area for naturally-occurring trona. There is also "synthetic soda ash", which is manufactured using a number of different chemical processes.
Soda ash is made in three main grades - light, medium and dense. These have the same chemical properties and only differ in physical characteristics, such as bulk density and particle size and shape (which affect flow characteristics and angle of repose).
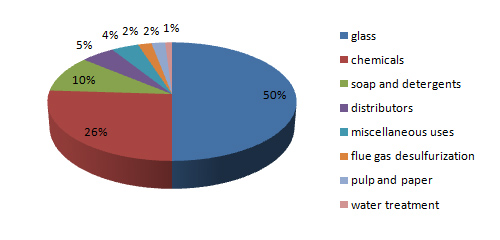
Application of Soda Ash
In terms of its largest applications, sodium carbonate is used in the manufacture of glass, paper, rayon, soaps, and detergents.
Glass manufacture
Sodium carbonate serves as a flux for silica, lowering the melting point of the mixture to something achievable without special materials. This "soda glass" is mildly water-soluble, so some calcium carbonate is added to the melt mixture to make the glass insoluble. Bottle and window glass (Soda-lime glass) is made by melting such mixtures of sodium carbonate, calcium carbonate, and silica sand (silicon dioxide (SiO2)). When these materials are heated, the carbonates release carbon dioxide. In this way, sodium carbonate is a source of sodium oxide. Soda lime glass has been the most common form of glass for centuries.
Water softening
Sodium carbonate is used to soften water by removing Mg2+ and Ca2+. These ions form insoluble solid precipitates upon treatment with carbonate ions:
Ca2+ + CO32- → CaCO3Sodium carbonate is an inexpensive and water-soluble source of carbonate ions.
Food additive and cooking
Sodium carbonate is a food additive (E500) used as an acidity regulator, anticaking agent, raising agent, and stabilizer. It is one of the components of kansui (かん水), a solution of alkaline salts used to give ramen noodles their characteristic flavor and texture. It is used in the production of snus to stabilize the pH of the final product. Sodium carbonate is used in the production of sherbet powder. The cooling and fizzing sensation results from the endothermic reaction between sodium carbonate and a weak acid, commonly citrix acid, releasing carbon dioxide gas, which occurs when the sherbet is moistened by saliva. In China, it is used to replace lye-water in the crust of traditional Cantonese moon cakes, and in many other Chinese steamed buns and noodles. In cooking, it is sometimes used in place of sodium hydroxide for lyeing, especially with German pretzels and lye rolls. These dishes are treated with a solution of an alkaline substance to change the pH of the surface of the food and improve browning.
Miscellaneous
Sodium carbonate is used by the brick industry as a wetting agent to reduce the amount of water needed to extrude the clay. In casting, it is referred to as "bonding agent" and is used to allow wet alginate to adhere to gelled alginate. Sodium carbonate is used in toothpastes, where it acts as a foaming agent and an abrasive, and to temporarily increase mouth pH.
Sodium carbonate is also used in the processing and tanning of animal hides.
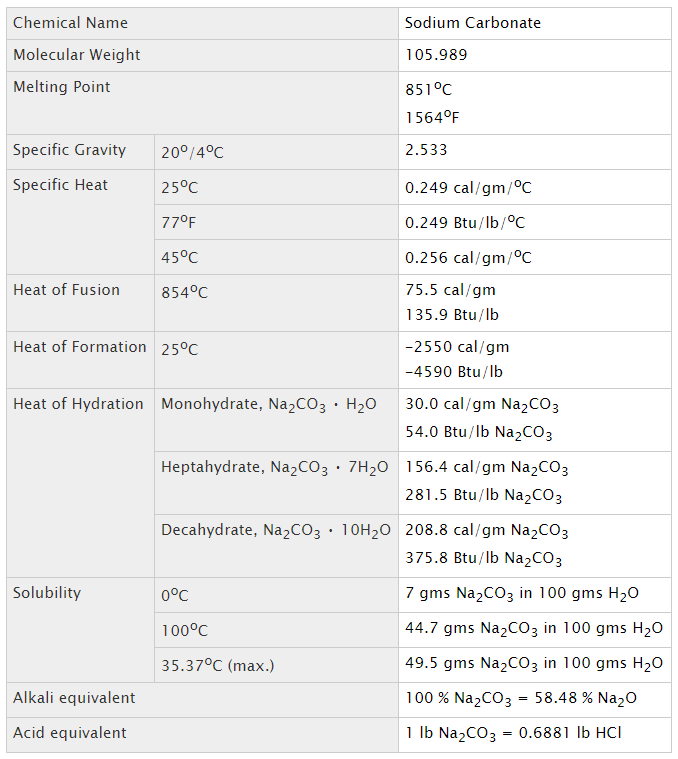
General chemical properties of soda ash




 contact
contact